Abstract
Background: Activated B-cell like diffuse large B-cell lymphoma (ABC-DLBCL) is a subtype of DLBCL, characterized by the constitutive activation of NF-κB and JAK/STAT3 pathways on the background of the chronic active BCR signaling. Being featured by mutations in MYD88 and/or CD79B, it potentially comprises the MCD/C5 subtype in the recent genetics-based categorization. In light of the inferior prognosis of this subtype to germinal center B-cell (GCB) like DLBCL, identification of subtype-specific strategies has been sought. Tumor progression locus 2 (TPL2) is an intracellular serine/threonine kinase that is reported to be activated downstream of TLRs/MYD88 signaling and to play roles in oncogenesis. However, the involvement of TPL2 in B-cell malignancies is less defined despite relatively higher transcript expression than in solid tumors.
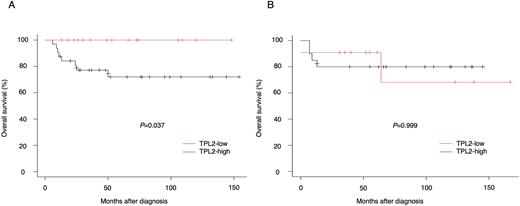
Results: To address the impact of TPL2 protein levels on DLBCL phenotypes, we performed an IHC analysis on 79 FFPE samples of DLBCL diagnosed and treated at our institution (48 non-GCB and 31 GCB according to Hans algorithm). When divided into two groups, one with strong TPL2 expression (TPL-high) and the other with no/weak expression (TPL2-low), the ratio of TPL2-high did not differ between non-GCB and GCB (67% vs 64%). However, TPL2-high had a significantly higher percentage of positive B symptom (42% vs 11%, p=0.005) and extra-nodal involvement (75% vs 52%, p=0.047) compared to TPL2-low, and these trends were observed more specifically in non-GCB. Moreover, it is noteworthy that TPL2-high showed a significantly lower overall survival in non-GCB (p=0.037), but did not in GCB (p=0.999) (Figure 1). Intriguingly, the analysis combined with pSTAT3 (Y705) staining revealed that TPL2-high accompanied by positive pSTAT3 tended to display particularly poor prognosis in non-GCB.
Then, we analyzed the mechanism by which TPL2 is involved in the tumorigenesis of ABC-DLBCL using TMD8, a well-described ABC-DLBCL cell line with MYD88L265P and CD79BY196 mutations. A specific TPL2 inhibitor significantly affected the cell proliferation of TMD8 cells, whereas did not on the cells with GCB phenotype. Of note, TPL2 inhibitor induced G1 cell-cycle arrest via downregulation of CyclinE and CDK2 and upregulation of p27 in western blot analysis, which was confirmed by quantitative PCR.
Next, we investigated the downstream signaling of TPL2 in ABC-DLBCL. Western blot analysis revealed that TPL2 inhibitor as well as genetic TPL2 knock down suppressed the activation of NF-κB components and STAT3, and the expression of c-MYC. These results implied the involvement of TPL2 in IL-6/IL-10 autocrine loop and resultant JAK/STAT3 activation. As expected, treatment with TPL2 inhibitor downregulated IL-6/IL-10 mRNA by quantitative PCR and accordingly decreased the levels of IL-6/IL-10 in the cell culture supernatant of treated TMD8 cells. Therefore, we speculated that TPL2 promoted the oncogenic activation of NF-κB and STAT3 pathways via IL-6/IL-10 upregulation in ABC-subtype.
Lastly, we evaluated the physiological function of TPL2 by western blot analysis using 293T. Although an overexpression TPL2 alone did not elicit NF-κB and STAT3 activation, when co-expressed with MYD88L265P TPL2 obviously activated these pathways, whereas dominant negative TPL2 failed to demonstrate those effects even with MYD88L265P co-expression. These findings indicated that TPL2 could possibly exert its tumorigenicity in MYD88L265P-dependent manner in ABC-DLBCL.
Conclusion: In association with the importance as an unfavorable prognostic factor, our results suggested that TPL2 might mediate the crosstalk between the oncogenic MYD88L265P and the activation of NF-κB and STAT3 pathways, coordinately contributing to the tumorigenesis of ABC-DLBCL. From this standpoint, TPL2 could emerge as a new therapeutic target in ABC-DLBCL.
Figure legend
Overall survival of non-GCB (A) and GCB (B) DLBCL patients, with regard to the TPL2 expression: TPL2-low (red line) or TPL2-high (black line). Overall survival was determined by the Kaplan-Meier method and compared by using the log-rank test.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal